Abstract
Background: Dysfunction of T cells, NK cells and other immune subsets is common in patients (pts) with CLL and Richter's transformation (RT). Venetoclax (VEN), a BCL-2 inhibitor and obinutuzumab (OBIN), a CD20 monoclonal antibody (mAb) have clinical activity in pts with DLBCL and RT (Davids, JCO 2017; Davids, ASCO 2020). Atezolizumab, a PD-L1 checkpoint inhibitor (CPI), is approved for melanoma, lung cancer and other solid tumors. Preclinical studies showed synergy of VEN and CD20 mAb with CPI (Kohlhapp, Cancer Discovery 2021; Westin, Lancet Oncology 2014). Clinical studies have shown activity of PD1 inhibition in pts with RT (Ding, Blood 2017; Jain, ASH 2018). To our knowledge, no prior study has evaluated PD-L1 inhibition in pts with RT and DLBCL histology.
Methods: This is an investigator-initiated Phase II trial of combined VEN, OBIN and atezolizumab in pts with RT (NCT02846623). Eligibility criteria included age ≥18 years, adequate organ function (total bilirubin ≤1.5 x ULN, ALT and AST ≤2.5 x ULN, creatinine ≤1.5 x ULN). Pts with either previously untreated or relapsed/refractory (R/R) RT were enrolled. Pts who received prior treatment with VEN were excluded. OBIN was given at a flat dose of 100mg IV Cycle (C)1 Day (D)1, 900 mg C1D2, 1000mg on C1D8, 1000mg on C1D15 and then 1000mg on C2-9 D1. Atezolizumab was given at a flat dose of 1680 mg IV (split over 2 days) on C1D3-4 and then C2-9D1-2. VEN was initiated at the start of C2 with weekly dose-escalation (20mg daily to a target dose of 800mg daily) and continued daily until end of C26 (total 24 cycles of VEN). Response assessments were done with PET imaging and bone marrow aspirate/biopsy with MRD assessment (multi-color flow cytometry; sensitivity 10 -4) at the end of C1 (prior to VEN initiation), end of C4, end of C9, and end of C26.
Results: From March 2020 to June 2021, a total of 8 pts were enrolled with DLBCL RT. The median follow-up is 11.2 months.
Newly diagnosed RT (n=7): Seven pts had newly diagnosed RT. The median age was 70 years (range, 52-80); 4 pts were ≥70 years; 4 pts were female. Previous therapy for CLL included ibrutinib, n=4; chlorambucil + OBIN and then acalabrutinib, n=1; BR, n=1; one pt had no prior therapy for CLL). CLL IGHV mutation status was available for 6 pts and all 6 were IGHV unmutated. CLL FISH panel showed del(17p) (n=4), del(11q) (n=1), trisomy 12 (n=1), and normal (n=1). Three pts had complex karyotype. Three pts had a TP53 mutation and 2 pts had a NOTCH1 mutation.
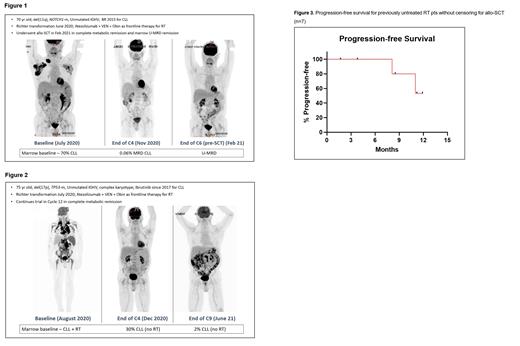
All 7/7 (100%) pts achieved a response (complete metabolic response, n=5; partial metabolic response, n=2). Three pts proceeded to an allogeneic stem cell transplant (allo-SCT) in complete metabolic remission after 4.1, 4.2 and 6.6 months; these 3 pts also achieved bone marrow undetectable (U)-MRD remission. One of these pts who went to allo-SCT has subsequently relapsed and is currently receiving a salvage regimen. One pt achieved partial metabolic remission and relapsed in C8, prior to a planned allo-SCT; this patient is now in remission after receiving a non-covalent BTK inhibitor. Three pts are continuing to receive treatment on the trial in C2, C5 and C12. Details about 2 pts including PET imaging and bone marrow findings are provided in Figures 1-2.
Majority of the responses were seen after the introduction of VEN in C2; however, 1 pt achieved complete metabolic response and bone marrow U-MRD remission after C1 with combined atezolizumab and OBIN (prior to VEN initiation).
Progression-free survival without censoring for transplant is shown in Figure 3. No pt has died.
One pt developed CPI-induced pancreatitis and diabetes mellitus. One pt required dose reduction of VEN to 400mg daily.
R/R RT (n=1): One pt (58-year-old male) with previously untreated CLL (unmutated IGHV, del(17p), TP53 mutation, NOTCH1 mutation) developed RT and received R-CHOP for 3 cycles with no response. The pt was subsequently enrolled on the current trial but did not respond.
Conclusions: Treatment with combined VEN, OBIN and atezolizumab leads to high rates of remission in pts with previously untreated RT; all 7 pts achieved a remission and 3 pts proceeded to allo-SCT. With the limitation of small numbers, these results are encouraging in relation to combined ibrutinib plus nivolumab in previously untreated RT (7/14, 50% response rate; Jain, ASH 2018). Enrollment in the trial continues and updated data will be presented at the ASH meeting.
Jain: TG Therapeutics: Honoraria; Janssen: Honoraria; Beigene: Honoraria; AbbVie: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pharmacyclics: Research Funding; ADC Therapeutics: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Incyte: Research Funding; Precision Biosciences: Honoraria, Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Servier: Honoraria, Research Funding; Pfizer: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding. Ferrajoli: BeiGene: Other: Advisory Board, Research Funding; AstraZeneca: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board . Thompson: Amgen: Other: Institution: Honoraria, Research Grant/Funding; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Gilead: Other: Institution: Advisory/Consultancy, Honoraria; AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Janssen: Consultancy, Honoraria. Konopleva: F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Stemline Therapeutics: Research Funding; Forty Seven: Other: grant support, Research Funding; KisoJi: Research Funding; AstraZeneca: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Cellectis: Other: grant support; Calithera: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding. Neelapu: Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene, Kuur, Incyte, Precision BioSciences, Legend, Adicet Bio, Calibr, and Unum Therapeutics: Other: personal fees; Takeda Pharmaceuticals and related to cell therapy: Patents & Royalties; Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, Acerta and Adicet Bio: Research Funding; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics and Bluebird Bio: Honoraria. Takahashi: Novartis: Consultancy; Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy; GSK: Consultancy. Strati: Astrazeneca-Acerta: Research Funding; Roche-Genentech: Consultancy. Burger: Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau. Khoury: Stemline Therapeutics: Research Funding; Angle: Research Funding; Kiromic: Research Funding. Kantarjian: Astra Zeneca: Honoraria; NOVA Research: Honoraria; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Astellas Health: Honoraria; Aptitude Health: Honoraria; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Wierda: Loxo Oncology, Inc.: Research Funding; Cyclacel: Research Funding; Miragen: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Janssen: Research Funding; Sunesis: Research Funding; KITE Pharma: Research Funding; Juno Therapeutics: Research Funding; Gilead Sciences: Research Funding; Acerta Pharma Inc.: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Karyopharm: Research Funding; Genentech: Research Funding; GSK/Novartis: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding; AstraZeneca: Research Funding; Xencor: Research Funding.
Atezolizumab is not approved for Richter transformation

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal